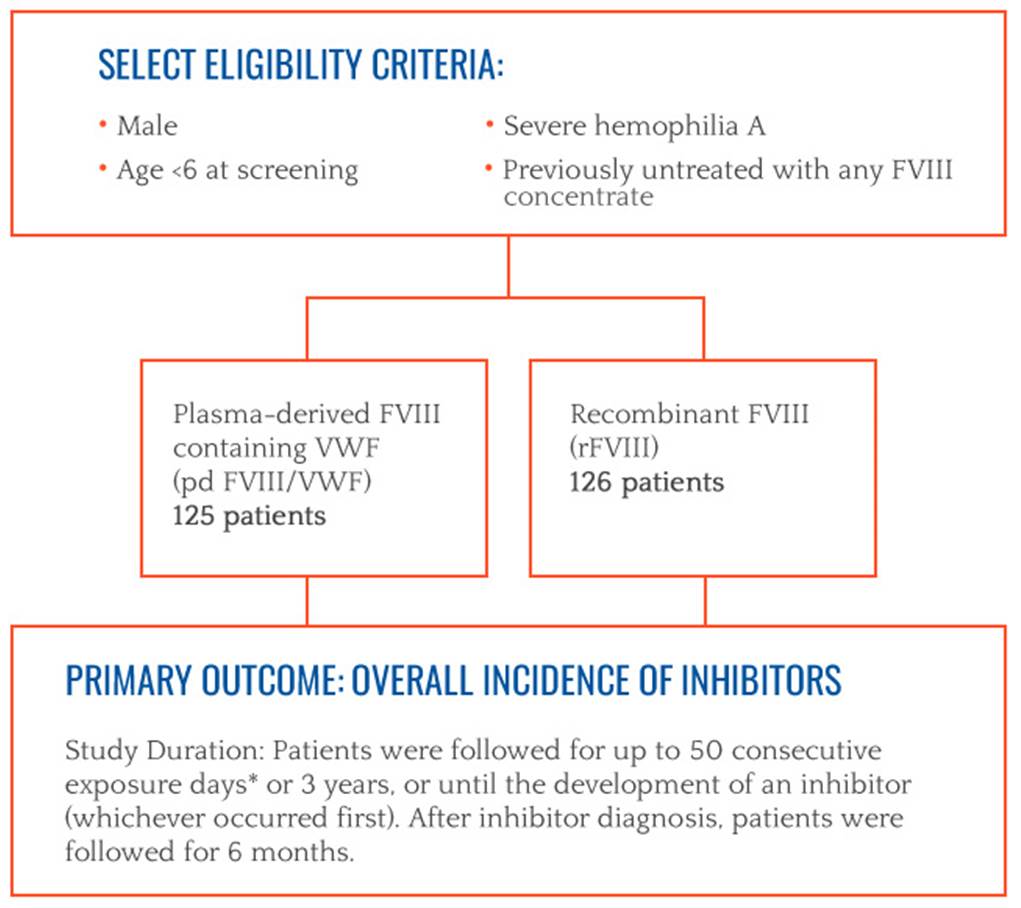
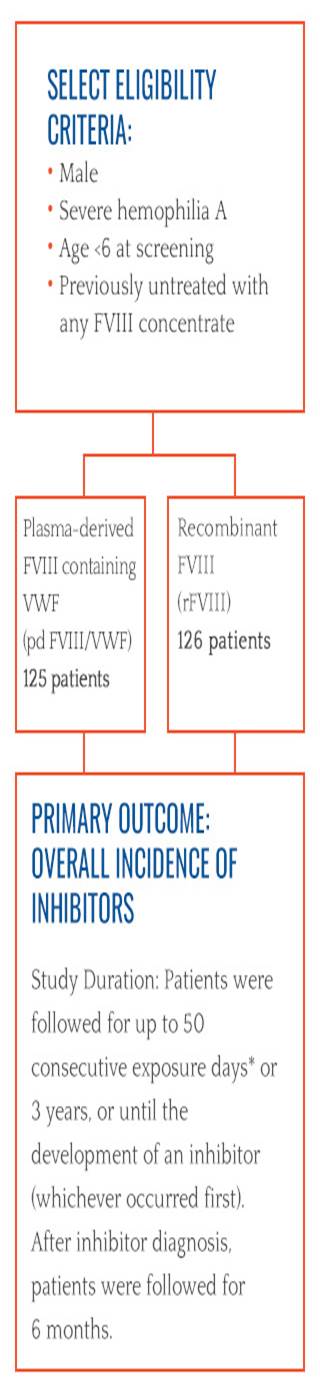
The SIPPET study answers an important question
Researchers have taken an important step toward understanding inhibitor development. Results from a randomized, controlled trial, called the SIPPET study, demonstrated that previously untreated patients with hemophilia A treated with recombinant factor VIII (FVIII) have a significantly higher risk of developing inhibitors compared to patients treated with plasma-derived FVIII containing von Willebrand factor (VWF).1
THE ROAD TO SIPPET: A HISTORICAL PERSPECTIVE
Over the years, medical research has tried to answer an important question: does the source of FVIII product used in replacement therapy impact the likelihood of inhibitor development? Studies have reported varying rates of inhibitor development with 2 types of FVIII products, plasma-derived and recombinant.1-4
Researchers Wight and Paisley reviewed the best evidence that was available in the published literature in 2003. Looking at 50 studies in previously untreated patients, they found high incidences of new inhibitor formation in previously treated patients started on some new products, and also from the lower overall cumulative incidence of inhibitors in PUPs treated uniquely with high- or intermediate-purity plasma-derived products compared with those treated uniquely with recombinant products.
CANAL stands for Concerted Action on Neutralizing Antibodies in severe hemophilia A. It was a retrospective cohort study that included 316 previously untreated patients with severe hemophilia A. CANAL concluded that the risk of inhibitor development was slightly lower with plasma-derived products compared with recombinant products. But the difference was not statistically significant.
RODIN stands for Research of Determinants of Inhibitor Development. It was a prospective observational study that included 574 previously untreated patients born between 2000 and 2010. Results showed no difference in the risk of developing inhibitors with plasma-derived FVIII vs recombinant FVIII. However, RODIN was not a randomized trial, and only 15% of the patients received plasma-derived FVIII.
THE QUESTION REMAINED UNANSWERED1
Experiments showed that plasma-derived FVIII products containing VWF may help reduce the risk of inhibitor development, but the observational studies that preceded SIPPET could not confirm this. These studies were uncontrolled, meaning that other risk factors and influences possibly biased the results, rendering them inconclusive. The SIPPET study was necessary to demonstrate if there was a difference in inhibitor development between recombinant FVIII and plasma-derived FVIII containing VWF in previously untreated patients.
SIPPET STUDY RESULTS1
The study demonstrated a significantly higher incidence of inhibitors with recombinant FVIII versus plasma-derived FVIII containing VWF. The rate of inhibitor development with recombinant FVIII was nearly doubles that with plasma-derived FVIII/VWF (HR=1.87 CI95:1.17 – 2.96). The authors concluded that these results may have implications on how physicians plan treatment in previously untreated patients with hemophilia A.
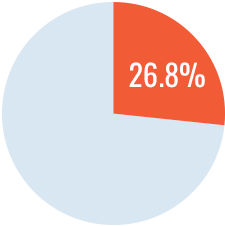
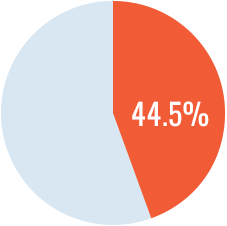
Cumulative incidence of inhibitor development1
Plasma-derived FVIII
125 patients
Recombinant FVIII
126 patients
 Patients with inhibitors
Patients with inhibitors
Recombinant FVIII had an 87% higher rate of inhibitor development than plasma-derived FVIII/VWF. (Cox regression HR=1.87, 95% CI: 1.17-2.96)1
IMPLICATIONS
Naturally occurring FVIII/VWF derived from human plasma resulted in significantly less inhibitor development in previously untreated patients than recombinant FVIII engineered in animal cell lines. This finding is clinically important because inhibitor development is the most severe complication in hemophilia A.1
Product choice matters with respect to inhibitor risk1
The SIPPET results may change the way doctors plan treatment, especially for previously untreated patients, the population studied.
Plasma-derived products containing VWF may be immunoprotective1
SIPPET results reflect some experimental studies showing that the presence of VWF in plasma- derived therapies may protect against inhibitor development.
Treatment Recommendations
Recommendations on plasma- derived FVIII vs recombinant FVIII may be adjusted to reflect these new findings on inhibitor development. Many organizations have adjusted their treatment recommendation based on these results.
Links to website:
http://www.ehc.eu/
http://www.wfh.org/
https://www.hemophilia.org/
http://www.ukhcdo.org/
IMPLICATIONS
Naturally occurring FVIII/VWF derived from human plasma resulted in significantly less inhibitor development in previously untreated patients than recombinant FVIII engineered in animal cell lines. This finding is clinically important because inhibitor development is the most severe complication in hemophilia A.1
Product choice matters with respect to inhibitor risk1
The SIPPET results may change the way doctors plan treatment, especially for previously untreated patients, the population studied.
Plasma-derived products containing VWF may be immunoprotective1
The SIPPET results reflect some experimental studies showing that the presence of VWF in plasma- derived therapies may protect against inhibitor development.
Treatment Recommendations
Recommendations on plasma- derived FVIII vs recombinant FVIII may be adjusted to reflect these new findings on inhibitor development. Many organizations have adjusted their treatment recommendation based on these results.
Links to website:
http://www.ehc.eu/
http://www.wfh.org/
https://www.hemophilia.org/
http://www.ukhcdo.org/
REFERENCES
- Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med. 2016;374(21):2054-2064.
- Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9:418-435.
- Gouw SC, van der Bom JG, Auerswald G, Ettinghausen CE, Tedgård U, van den Berg HM for the CANAL Study Group. Recombinant versus plasma-derived factor VIII products and the development of inhibitors in previously untreated patients with severe hemophilia A: the CANAL cohort study. Blood. 2007;109(11):4648-4654.
- Gouw SC, van der Bom JG, Ljung R, et al, for; the PedNet and RODIN Study Group. Factor VIII products and inhibitor development in severe hemophilia A. New Engl J Med. 2013;368(3):231-239.
- National Hemophilia Foundation. MASAC Update on SIPPET. National Hemophilia Foundation website. https://www.hemophilia.org/Newsroom/NHF-Community-News/MASAC-Update-on-SIPPET. Published March 9, 2016. Accessed May 25, 2016.