Clinical studies on ITI
Studies with pdFVIII/VWF concentrates

"Immune tolerance induction with a high purity von Willebrand factor/VIII complex concentrate in haemophilia A patients with inhibitors at high risk of a poor response."1
Gringeri A. Haemophilia 2007
- Objective: To identify the effectiveness in ITI of a high purity pdFVIII/VWF concentrate in inhibitor patients at high risk of failure.
-
Design: Prospective, observational, multicenter study.
- Enrolled patients: 16 severe HA; 1 moderate HA.
- Aged 4–54 years.
- Followed-up for 6–71 months.
-
Results:
- Overall success rate* of 94% (complete success + partial success)
- 53% complete success and 41% partial success

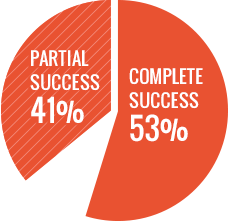
*Complete success: <0.5 BU on at least 2 consecutive monthly assessments and confirmed by a central lab, and recovery and half-life >66% of expected values
Partial success: inhibitor peak not rising above 3 BU and/or FVIII recovery and half-life <66% of expected values.
Failure: reduction in BU <20% within 9 months of the anamnestic response peak during ITI.

"The use of a single von Willebrand factor-containing, plasma-derived FVIII product in hemophilia A immune tolerance induction: the US experience."2
Kurth M. Thromb Haemost. 2011
- Objective: To report retrospective data on the use of a single pdFVIII/VWF concentrate in primary and rescue ITI.
- Design: Retrospective, observational, investigator-led multi-center study.
-
Results:
-
Success rates*
- - Primary ITI in 8 patients: success rate of 75%.
- - Secondary ITI in 25 patients: success rate of 52%.
-
Success rates*
Primary ITI Success
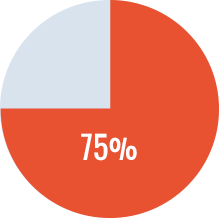
*Complete success: <0.6 BU, ≥66% recovery, and half-life >6 hr
Partial success: <5 BU, with ability to FVIII product for the treatment of bleeding episodes without anamnesis.
Failure: less than 20% reduction in titer in any 6-month period after the initial 3 months of treatment and/or failure to fulfill criteria for either partial or complete success.

"Primary and rescue ITI in children and adults: a multicenter international study with a VWF-containing plasma-derived FVIII concentrate."3
Oldenburg J. Haemophilia 2014
- Objective: To report the outcome of patients who underwent ITI treatment with a pdFVIII/VWF concentrate.
-
Design: International, multicenter, observational, retrospective study.
- Enrolled patients: 60 hemophilia A patients (FVIII < 2%).
- Primary ITI in 41 patients: 32 children and 9 adults.
- Rescue ITI in 19 patients: 17 children and 2 adults.
-
Results:
-
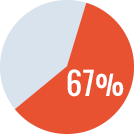
Overall success rate* of 84% (complete + partial success)
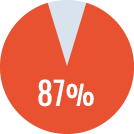
- - Primary ITI in 41 patients: success rate of 87%
- - Rescue ITI in 19 patients: success rate of 67%
-
Overall success rate* of 84% (complete + partial success)
Overall success
Rate
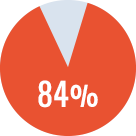
Primary ITI
Success
Secondary ITI
Success
*Complete Success: <0.6 BU at 33 months, recovery ≥66% and half-life at ≥6 hrs.
Partial Success: <5 BU, recovery <66% and half-life <6 hrs associated with clinical response to FVIII not followed by a treatment limiting anamnestic rise >5 BU
Failure: inability to fulfill the criteria for CS or PS.

"Adult hemophilia A patients with inhibitors: successful ITI with a single FVIII/VWF product"4
Rangarajan S. Haemophilia 2014
- Objective: To evaluate ITI in an adult cohort.
- Design: Observational, retrospective study on 20 adult patients.
-
Results:
-
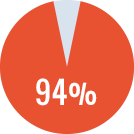
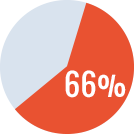
Overall success* rate of 90%
- Primary ITI in 17 patients: success rate of 94%
- Rescue ITI in 3 patients: success rate of 66%
-
Overall success* rate of 90%
Overall success
Rate

Primary ITI
Success
Secondary ITI
Success
*Complete Success: Having undetectable inhibitor titer (<0.6 Bethesda Units [BU]) at 33 months of ITI, FVIII recovery ≥66% and half-life ≥6 h;
Partial Success: having reduction in inhibitor titer to <5 BU/mL_with FVIII recovery <66% and/or FVIII half-life <6 h associated with clinical response to FVIII therapy not followed by a treatment-limiting anamnestic rise in inhibitors to >5 BU/mL.
Failure: inability to fulfill the criteria for CS or PS.

"First prospective report on immune tolerance in poor risk haemophilia A inhibitor patients with a single factor VIII/von Willebrand factor concentrate in an observational immune tolerance induction study"5
Kreuz W. Haemophilia 2016
- Objective: To evaluate the success on ITI, the standard of care in patients with inhibitors and risk factors.
-
Design: Ongoing, international, prospective, open-label, uncontrolled, observational ITI.
- Enrolled patients: 48 patients
-
Results:
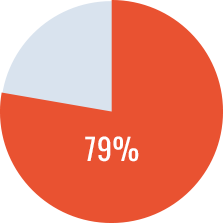
- 40 of 48 patients (83.3%) had at least one risk factor for poor ITI-prognosis at ITI start.
- 34 patients (70.8%) achieved complete success*, 3 (6.3%) partial success**, 1 (2.1%) partial response***; ITI failed**** in 10 patients (20.8%), all with poor prognosis factors.
- All low responders achieved complete success.
Overall success rate
*Complete Success (CS): Required achievement of all three criteria.
**Partial success (PS): Required achievement of two
***Partial response (PR): Required achievement of one of the three criteria.
****Failure: If no criteria were met within the 36-month observation period, i.e. presence of a persistent Inhibitor.
The three efficacy criteria:
- Criterion I – inhibitor titer <0.6 BU;
- Criterion II – FVIII recovery ≥80% of the predefined reference value of 1.5% IU-1 kg-1 bw ≤1 h post injection;
- Criterion III – FVIII half-life ≥7 h.

"Long-Term Outcome of Haemophilia A Patients After Successful Immune Tolerance Induction Therapy using a Single Plasma-Derived FVIII/VWF Product: The Long-Term ITI Study"6
Jimenez-Yuste V. Haemophilia 2016
- Objective: To perform a long-term follow-up to describe the status of patients reported as ITI success in the Grifols ITI Study3
-
Design: International, multicenter, observational, retrospective study.
- Enrolled patients: 44 eligible for the follow up
-
Results:
- The mean follow up period after ITI success* was 9.3±3.3 years (range: 2.1-17.7).
- The clinicians' main treatment of choice for the post-ITI patients was prophylaxis (>93% of patients).
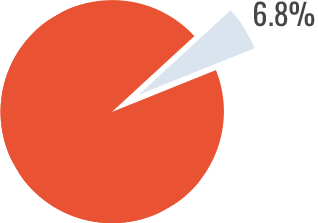
- Only 3/44 patients (6.8%) showed inhibitor relapse.
Inhibitor Relapse
Studies with rFVIII concentrates

"Immune tolerance induction with recombinant factor VIII in hemophilia A patients with high responding inhibitors."7
Rocino A. Haematologica 2006
-
Objective:
- To evaluate the success rate of ITI using rFVIII concentrates in patients with severe hemophilia A and high-responding inhibitors.
- To obtain data on the relationship between F8 gene mutations and ITI outcome and evaluate other factors influencing outcome.
-
Design: Observational, multicenter study on 26 patients that underwent ITI.
- The ITI regimens ranged from 50 FVIII IU/Kg every other day to 200 IU/kg kg-1 daily, with 17 patients (65%) receiving 100 IU/Kg daily.
-
Results:
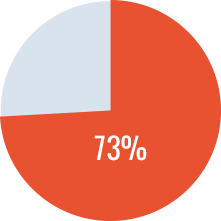
- Success rate* of 73%
- Success rate of 70% for patients with intron 22 inversion
Overall Success Rate
*The concomitant presence of the following criteria was used to define the ITI as successful: no detectable inhibitory activity; normalization of in vivo FVIII recovery (> 66%); half-life of infused FVIII > 6 hrs.
Partial success: no detectable inhibitor activity without normalization of the in vivo recovery (< 66%) and half-life (< 6 hrs) of FVIII.
Failure: no decrease of the inhibitor over a 6-month period after the first 3 months of ITI.

"Experience with a third generation recombinant factor VIII concentrate for immune tolerance induction in patients with haemophilia A."8
Valentino LA. Haemophilia 2009
- Objective: To evaluate the success rate of ITI in 12 children with severe hemophilia A who underwent ITI using a third-generation rFVIII concentrate over a period of 30 months.
- Design: Retrospective chart review.
-
Results:
- Overall success rate* of 75%
- 70% success rate among patients with high-titer inhibitors
- 100% success rate among patients with low-titer inhibitor
Overall Success Rate

*Successful Tolerance: an undetectable inhibitor level (<0.6 BU mL) 1), FVIII plasma recovery ≥66% of predicted, FVIII half-life of ≥6 h after a 72-h FVIII washout period and the absence of anamnesis upon further FVIII exposure.

"Principal results of the International Immune Tolerance Study: a randomized dose comparison"9
Hay CR. Blood 2012
- Objective: To test the hypothesis that overall response to ITI is independent of FVIII dosing regimen in good-risk subjects and to assess reported prognosis for success of ITI outcome
-
Design: Multicenter, prospective, randomized study to compare High-dose (HD) and Low-dose (LD) ITI in 115 randomized patients.
- All patients were ≤ 8 years with severe hemophilia A and high-titer inhibitors.
- rFVIII was used in 90% of the cases and pdFVIII/VWF concentrate in 10%.
-
Results:
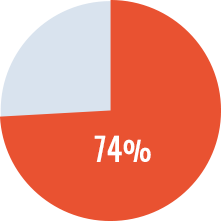
- Overall success rate* of 74%. Successes did not differ between treatment arms.
-
LD had increased bleeding complications compared with HD.
Early bleed rate/month was 0.62 (LD) and 0.28 (HD), decreasing by 90% once negative titers were achieved. -
o HD achieved negative inhibitor and normalized FVIII recovery earlier than LD.
Success rate average time was better for HD patients 14.2 months rather than in LD ones 16.4 months
Overall Success Rate
*Successful Tolerance: negative BU titer, recovery ≥66% and half-life ≥6 hrs.
Partial Response: after 33 mo of ITI, negative titer but persistently abnormal recovery or half-life; responding clinically to FVIII replacement without an anamnestic increase in inhibitor titer.
Failure: failure of BU to decline by ≥20% over any 6-month period after the first 3 months if ITI, or failure to achieve tolerance or partial response after 33 months on ITI, or withdrawal for any reason before tolerance was achieved.
It is important to note that treatment varies by patient. Be sure to consult a healthcare professional before making any treatment decisions and continue to remain informed to help facilitate care.
REFERENCES
- Gringeri A.et al. Immune tolerance induction with a high purity von Willebrand factor/VIII complex concentrate in haemophilia A patients with inhibitors at high risk of a poor response. Haemophilia 2007 Jul; 13(4):373-9.
- Kurth M. et al. The use of a single von Willebrand factor-containing, plasma-derived FVIII product in hemophilia A immune tolerance induction: the US experience. J. Thromb Haemost. 2011 Nov; 9(11):2229-34.
- Oldenburg J. et al. Primary and rescue ITI in children and adults: a multicenter international study with a VWF-containing plasma-derived FVIII concentrate. Haemophilia 2014 Jan; 20(1):83-91.
- Rangarajan S. et al. Adult haemophilia A patients with inhibitors: successful immune tolerance induction with a single FVIII/VWF product. Haemophilia 2014, 20, e399-e443.
- Kreuz W.et al. First prospective report on immune tolerance in poor risk haemophilia A inhibitor patients with a single factor VIII/von Willebrand factor concentrate in an observational immune tolerance induction study. Haemophilia 2016 Jan;22(1):87-95.
- Jimenez-Yuste V. et al. Long-Term Outcome of Haemophilia A Patients After Successful Immune Tolerance Induction Therapy using a Single Plasma-Derived FVIII/VWF Product: The Long-Term ITI Study. Haemophilia 2016. DOI: 10.1111/hae.12986
- Rocino A. et al. Immune tolerance induction with recombinant factor VIII in hemophilia A patients with high responding inhibitors. Haematologica 2006 Apr;91(4):558-61
- Valentino LA. et al. Experience with a third generation recombinant factor VIII concentrate for immune tolerance induction in patients with haemophilia A. Haemophilia 2009 May; 15(3):718-26.
- Hay CR. et al. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood 2012 Feb 9; 119(6):1335-44.
