Reflections on SIPPET
The SIPPET study reignited the debate about the optimal class of FVIII concentrates to limit inhibitor development, particularly in previously untreated patients (PUPs) in the United States. Here are some of the implications and conclusions we can draw through the largest hemophilia A study conducted yet. Come back to this page often, it will be updated with the latest analyses of this pivotal trial.
Investigating the Influence of SIPPET on Clinical Practice1
In light of the ongoing discussion in the hemophilia community about the implications of SIPPET, the National Institutes of Health (NIH) funded surveys of expert hemophilia physicians about SIPPET and its influence on their clinical practice in the United States, which were published in 2019.
Study design
A nationwide, cross-sectional web-based study of expert hemophilia physicians who self-identified as US hemophilia treaters

Conducted via 2 anonymous, voluntary, multiple choice surveys, where physicians answered based on their current clinical practice

Survey sections included demography, standard of care, SIPPET publication, and clinical vignettes
Survey 1 focused on the initial SIPPET publication and was conducted after its publication, from December 2016 to February 2017. Fifty-eigh percent (76/130) of participants responded to the survey, with 80% (61/76) completing it.
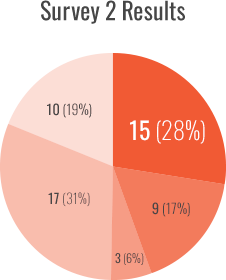
Survey 2 focused on the SIPPET post hoc analyses and was conducted after its publication, from June 2018 to August 2018. Fifty-six percent (73/130) of participants responded to the survey, with 74% (54/73) completing it.
RESULTS SHOWED SIPPET MADE AN IMPACT ON CLINICAL PRACTICE
Use of recombinant FVIII (rFVIII) as the standard of care dropped to less than a third while the use of plasma-derived FVIII containing von Willebrand factor (pdFVIII/VWF) more than doubled:
- rFVIII selection decreased from 70% to 28% during the ~20-month study period (P<0.001, 43/61 and 15/54, respectively)
- pdFVIII/VWF selection increased from 8% to 17% during the ~20-month study period
- A risk-stratified approach to treatment selection increased from 7% to 31%
- Adopting shared decision-making to treatment selection increased from 7% to 19%
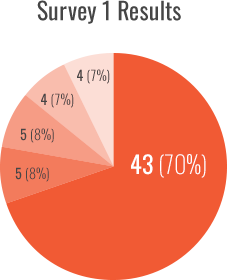

77% of hemophilia experts who participated in Survey 1 indicated that the SIPPET study would influence their clinical practice (47/61)
CONCLUSIONS
According to the surveys:
- The SIPPET study and its post hoc analyses have influenced clinical practice of treating PUPs in the United States
- The use of rFVIII as standard of care dropped from the majority of participants to less than a third while the use of pdFVIII/VWF more than doubled
- The use of risk-stratified and decision-sharing approaches increased (especially for the use of pdFVIII/VWF for low-risk PUPs)
- Nearly a third of clinicians had developed institutional practice guidelines to streamline their approach to treating PUPs, with the majority of guidelines developed after the initial SIPPET publication
82% of hemophilia experts who participated in Survey 2 would change their clinical practice based on SIPPET (44/54)
The results of the 2 surveys indicate that the SIPPET study and its post hoc analyses have influenced clinical practice in the United States, particularly with regard to considering pdFVIII/VWF for PUPs
STAY CONNECTED
Sign up to receive access to the full SIPPET study, as well as updates about hemophilia, inhibitors, and the latest clinical data.
REFERENCE
- Sande CM, Al-Huniti A, Ten Eyck P, Sharathkumar AA. Impact of the Survey of Inhibitors in Plasma-Product Exposed Toddlers (SIPPET) study and its post hoc analyses on clinical practice in the United States: a survey of Haemophilia and Thrombosis Research Society members. Haemophilia. 2019;25(5):764-772.
